Point-of-care REGEN-COV™ Infusion using the co-formulated vial and Vial2Bag Advanced® Admixture Device Maximizes Efficiency
The Pfizer-BioNTech COVID-19 vaccine, now marketed as Comirnaty received FDA approval this week for the prevention of COVID-19 disease in individuals 16 years of age and older, marking another pivotal moment in this fight, and the timing could not be more critical. Infections and hospitalizations are rising as the highly contagious delta variant spreads across the U.S. and Healthcare Systems are overloaded with patients desperate for care. A new COVID-19 post-exposure prophylaxis (PEP) medication REGEN-COV™ was authorized for emergency use by the FDA at the end of last month and outpatient clinics are managing unprecedented surges in patients prescribed the powerhouse drug combination (Figure 1. FDA EUA mAb Therapies in Outpatient COVID-19 Timeline). Some state Health Departments are opening COVID antibody infusion centers and deploying the National Guard medical staff and others are issuing Standing Orders authorizing additional eligible healthcare providers to administer the medications. A highly transmissible variant of the virus circulating in our communities, only 60% U.S. vaccination rates and waning vaccine immunity means more patients infected and increasing demands on an overflowing system. Amid staffing shortages and IV infusion supply deficiencies, clinicians are utilizing Vial2Bag Advanced® Admixture Device, a point-of-care admixture device, to maximize operational efficiency. Miracle drugs and vaccines are available and state health departments are stepping in to help overburdened hospitals and clinics, but the lines of patients are an ominous view. Improved vaccination rates, booster dose access, brilliant treatment options, and the dedication of the healthcare heroes continue to offer hope.
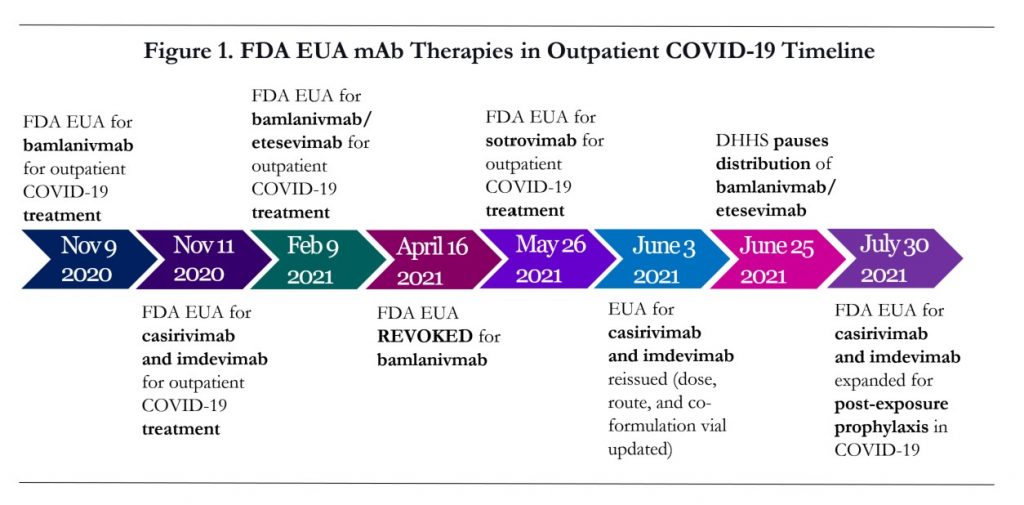
REGEN-COV™ is the combination of casirivimab (kas i RIV i mab) and imdevimab (im DEV i mab) made by Regeneron®. The FDA expanded emergency use authorization (EUA) for prevention after exposure to SARS-CoV-2 virus includes adults and children ≥12 years of age (weighing ≥40 kg) who are at risk for severe COVID-19 disease (Table 1: Criteria for Identifying Individuals at High Risk for Severe COVID-19 Disease) and who are not fully vaccinated, or who are not expected to build up enough of an immune response to vaccination, or who are at high risk for exposure to an infected individual.

Casirivimab and imdevimab are monoclonal Antibody (mAb) medications or immune-modulators used to modify or regulate immune functions. mAbs are engineered versions of antibodies that are naturally produced by the immune system in response to an invasion of the virus. The SARS-CoV-2 specific mAbs target part of the infamous spike protein of the virus, preventing it from entering human cells and inhibiting viral replication. REGEN-COV™ received FDA EUA for outpatient treatment of COVID-19 in November 2020. In June 2021, the EUA was updated to include a co-formulation vial (both drugs in single vial), updated dosage, and authorizing sub-Q injection as alternative when IV route is not feasible. But the July 30, 2021 EUA expansion to include post-exposure prophylaxis elevates this medication to a new realm, joining the COVID-19 vaccines as an important medication of our time, and for good reason. The New England Journal of Medicine study published August 4, 2021 indicates that the combination cocktail REGEN-COV™ reduced symptomatic COVID-19 infection 81% in patients exposed to a COVID-19 patient. When including asymptomatic infections, the relative risk reduction for REGEN-COV™ therapy was 66.4%. COVID-19 infections in the patients receiving REGEN-COV™ had milder illness with shorter duration of symptoms and were less likely to have prolonged high viral load. The data is promising for REGEN-COV™ but there are limitations that must be considered. Post-exposure prophylaxis with REGEN-COV™ is NOT a substitute for vaccination against COVID-19 and is not authorized for pre-exposure prophylaxis.
SPECIAL CONSIDERATIONS: Post-exposure prophylaxis with REGEN-COV is NOT a substitute for vaccination against COVID-19 and is not authorized for pre-exposure prophylaxis
REGEN-COV™ post-exposure dosage is 600 mg of casirivimab and 600 mg of imdevimab administered together as a single intravenous infusion (alternative is subcutaneous injection) as soon as possible following exposure to SARS-CoV-2. The medications are available in two separate drug vials and as a co-formulation vial. The drugs can be diluted in 50, 100, 150 and 250 ml IV bag volumes and the infusion time increases with the bag volume, ranging from 20 minutes to 50 minutes with an additional hour of clinical monitoring after completion (TABLE 2. REGEN-COV™ IV Infusion Bag Volume and Infusion Durations). The U.S. Department of Health and Human Services (HHS) and the Department of Defense (DOD) have purchased mass quantities of REGEN-COV™ and are providing it at no cost to patients, though healthcare facilities may charge an administration fee.

The REGEN-COV™ IV infusion can be prepared in advance in a Pharmacy IV Clean Room or using the co-formulated vial, assembled by the nurse immediately prior to starting the infusion with Vial2Bag Advanced® Admixture Device , a universal* admixture device or admixture system. Vial2Bag Advanced® Admixture Device serves as a connection between a drug vial (with standard 20mm diameter, liquid and powder drugs) and ANY manufacturers brand IV bag up to 250ml. Utilization of an admixture device that allows for IV bag flexibility is critical in times of increased demand and likely supply disruptions. The needle-free device is designed for simple point-of-care medication preparation, improving timeliness of medication delivery while maximizing pharmacy IV clean-room operational efficiencies.
Use of Vial2Bag Advanced® Admixture Device inside the hospital for point-of-care medication preparation was evaluated in a study published in 2019** reviewing the cost, workflow and safety of implementing the device compared to local pharmacy compounding and purchase of ready-to-use infusions. Implementing the Vial2Bag Advanced® Admixture Device system resulted in an estimated yearly cost avoidance of $2,295,261 and 41,082 yearly sterile product room units were avoided. With the June availability of the REGEN-COV™ co-formulation vial, Vial2Bag Advanced® Admixture Device can be used for point-of-care medication preparation, eliminating pharmacy compounding time and ensuring the medication is prepared as close to the time of administration as possible, minimizing sterility risks and maximizing the number of patients that can be seen.
When patients are receiving IV antibody infusions from the floor of gymnasiums and libraries, and the number of healthcare workers available to treat and care for our sick is dwindling as the trauma of the last 18 months’ settles, the SARS-CoV-2 virus ravages on. The demand for COVID-19 treatment is high and healthcare resources are finite. Surges in COVID-19 patients are occurring across the country and small wins in operational efficiency with IV infusion technology can help more patients receive life-saving care. The gap in the resources available and the patients needing them is growing and our healthcare clinicians are exhausted. As the government allocates resources and monumental advances in medicine take place, the diligent service of our healthcare providers remains constant and again carries us forward in this battle against SARS-CoV-2. If you want to help the heroes caring for the sick and dying, or to honor those that have lost their lives to COVID-19, or maybe you just don’t want to die from this disease, please roll-up your sleeves, and get vaccinated. The battle cry from your healthcare clinicians continues and there are only so many ways to take care of more people with less resources. Protect your health and wellness. Talk to your trusted healthcare provider today about how you can live well.
*Vial2Bag Advanced® 20mm Admixture Device is compatible with manufacturers’ IV bags in 50mL, 100mL, or 250mL that contain an ISO standard IV port; ISO 8536-4 standard IV spike
**Vial2Bag® admixture device and Vial2Bag Advanced® admixture device are separate products; West’s Vial2Bag Advanced® admixture device replaced the Vial2Bag® admixture device in commerce.
Prescription Use Only.
The Vial2Bag Advanced® 20mm admixture device is 510(k) cleared by the United States Food and Drug Administration (FDA). The use of the Vial2Bag Advanced® 20mm admixture device should not be interpreted as modifying, extending, or superseding a drug manufacturer’s labeling recommendations for storage and expiration dating, unless otherwise limited by USP <797> compounding standards. Refer to drug manufacturer’s labeling and use instructions for recommendations, USP <797>, and applicable institution policy for shelf life and sterility information of reconstituted product and admixture device compatibility. The Vial2Bag Advanced® 20mm admixture device is not compatible for use with all drug products. Do not use the Vial2Bag Advanced® 20mm admixture device with lipids. Failure to follow the instructions provided may result in inadequate medication reconstitution, dilution, and/or transfer, possibly leading to overdose or underdose and/or delay in therapy. Products shown are for INFORMATION purposes only and may not be approved for marketing in specific regions. Important product and safety information and warnings at: https://www.westpharma.com/products/vial-adapter-systems/vial2bag-advanced.
West and the diamond logo are registered trademarks of West Pharmaceutical Services, Inc. in the United States and other jurisdictions. Vial2Bag Advanced® and logo and Blue Vial Adapter are registered trademarks of West Pharma. Services IL, Ltd., a subsidiary of West Pharmaceutical Services, Inc.


